Work with us
We partner with drug developers to transform complex biologic formulation challenges into opportunities for success.
Case studies
We customize formulations to address the specific delivery, storage and administration requirements of each partner.
Integrate multiple components into a single, stable product
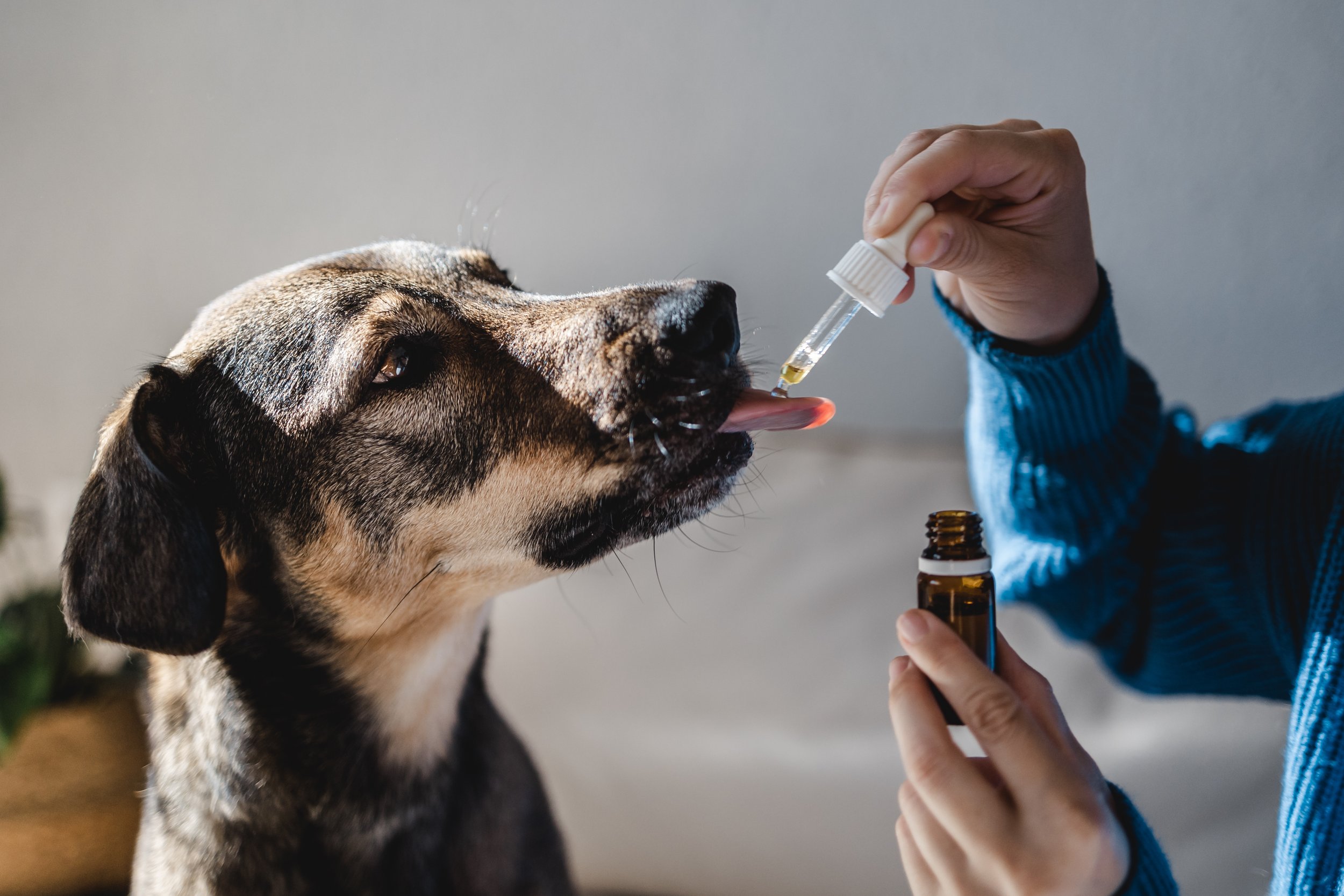
Although Jurata’s technology has demonstrated promise across diverse payloads, our partners often focus on vaccine applications because of the critical global need and pervasive present-day challenges. One veterinary partner tested a multivalent vaccine in a liquid presentation for canines, achieving antibody titers that matched discreet formulations. This approach has potential to streamline production, reduce manufacturing complexity, and improve overall efficacy. In veterinary applications, this creates the potential to combine multiple formerly injected vaccines into a single chewable format.
Break the cold chain, without compromising quality or efficacy.
In our work with a team developing an Ebola vaccine, film doses maintained efficacy in challenge experiments after 30 days of storage at room temperature, and outperformed intramuscular injection. In further studies, adenoviral vectors remained stable in films for at least three years at room temperature; rehydrated films were stable for at least eight months. This level of thermostability means that complex biologics no longer need to be stored at low or ultralow temperatures, removing the need for refrigeration during storage or transport and freeing patients who use medications such as Humira or insulin from the burden of ice packs and coolers.
Create global access to breakthrough medicines and vaccines
Lipid nanoparticles (LNPs) played a pivotal role in rapid COVID-19 vaccine development, yet demanding cold-chain requirements restricted global access. In collaboration with our partners, we are working to formulate mRNA-LNPs into thermostable presentations that eliminate the need for frozen storage. These presentations will simplify transportation logistics, lower shipping costs, and permit higher-volume shipments. Our partners see this effort as a way to expand vaccine availability in remote or resource-limited areas that lack the necessary refrigeration infrastructure; Jurata’s technology could also be applied to LNPs in other settings that may benefit from reduced handling requirements.